July is UV Safety Month - click here for FREE healthy vision tips
Menu

Can-C™ Eye Drops – Approved by Innovative Vision Products”
Two (5ml) vials per box
Can-C™ acts as both a stabilizer and carrier for safe delivery into the aqueous humor of the eye. Once delivered into the aqueous humor this bio-identical molecule becomes highly active.
Can-C™ Ingredients:
Excipients:
Dosage: Apply 4 drops daily to each affected eye.
Each carton of Can-C™ contains two (5ml vials) and each 5ml vial contains approximately 65-drops. Applying two drops to each eye, morning and evening, one box of Can-C™ will last approximately three weeks. (NOTE: Will last 6 weeks if treating one eye)
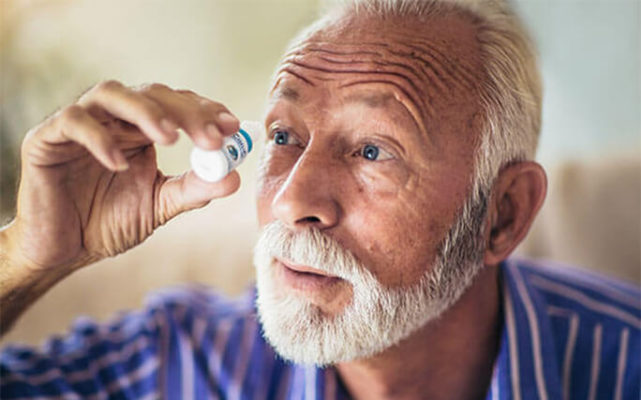
Eye Drop Application Instructions:
Instructions on applying drops to dogs: click here for article
Storage: Keep in cool dry environment and do not expose to extreme temperatures. All open vials should be discarded after 30 days.
Can-C™is Manufactured in a GMP (Good Manufacturing Procedures) certified, pharmaceutical facility which meets ISO 9001:2000 and ISO 13485:2003 standards for the design/formulation and manufacture of sterile contact lens solution and pharmaceutical solutions per FDA guidelines. Only the lubricants (Carboxymethylcellulose sodium) and (glycerin) are approved by the FDA for ophthalmic use, and can be listed as active ingredients on our label. Can-C™ is not FDA approved for the treatment of cataracts therefore N-Acetylcarnosine (1%) is listed as an inactive ingredient on the label.
Disclaimer: The above information is presented for educational purposes only and does not replace the advice of your physician.
Free shipping on all US orders
Your data is safe
Satisfaction guaranteed
Email support is always available

*Disclaimer: The above statements have not been evaluated by the FDA. They are not intended to diagnose, treat, cure or prevent any disease or condition.
If you have a health condition or concern, consult a physician or your alternative health care provider.
Always consult a medical doctor before modifying your diet, using any new product, drug, supplement, or doing new exercises.
Use of this site constitutes acceptance of our Shipping and Returns Policy, our Terms and Conditions and our Privacy & Cookies Policy
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |
Any questions? We’re here to help.